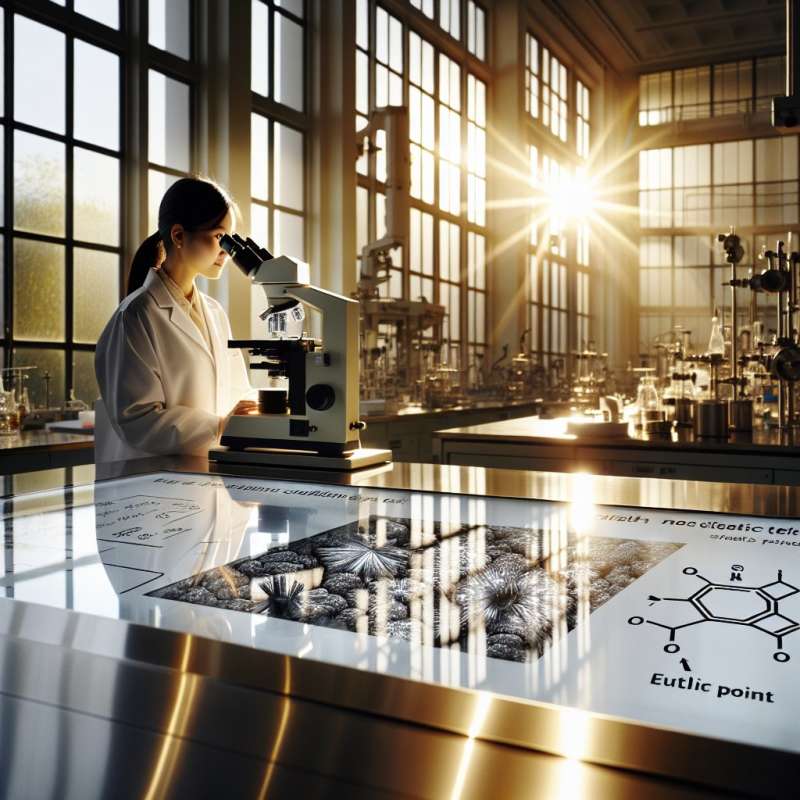
Eutectic Solidification Introduction
Eutectic solidification occurs when a liquid solution solidifies into two or more distinct phases at a specific composition and temperature, known as the eutectic point.
Eutectic System Basics
In a eutectic system, the components have limited solubility in each other's solid phase. The eutectic reaction involves simultaneous crystallization of the phases from the melt at a constant temperature.
Coupled Growth Phenomenon
During eutectic solidification, coupled growth refers to the coordinated formation of two distinct solid phases. These phases grow cooperatively due to atomic scale interactions and compositional undercooling.
Lamellar and Rod Structures
Eutectic structures often form lamellar or rod-like patterns. The lamellar structure, consisting of alternating layers, maximizes interface stability, while rod structures form when one phase grows preferentially.
Importance of Interface Stability
Interface stability during eutectic solidification dictates the microstructure and properties of the final material. Unstable growth can lead to eutectic colonies and irregular microstructures.
Eutectic Microstructure Control
Controlling eutectic microstructures is critical for materials with specific properties. Factors like growth rate, alloy composition, and temperature gradients are manipulated to tailor the microstructure.
Applications of Eutectic Alloys
Eutectic alloys are vital in various applications due to their unique properties. They are used in soldering, brazing, casting and even in thermal storage materials due to their sharp melting point.
What defines a eutectic point?
Constant temperature, composition change
Multiple phases, varying temperature
Specific composition, constant temperature
Company